Technical Feasibility

A very exciting, even seemly promising, idea, and/or a cutting-edge technology, needs to be carefully flushed out with design considerations, theoretical modeling, initial risk assessment, and rigorous testing and adjusting, before it becomes technically feasible. Expertise in basic sciences such as math, physics and chemistry, as well as applied sciences including human biological sciences, design, engineering, and so on, needs to come together to make a design comprehensible and technically feasible.
Helical Sphere is here to guide you through the maze for the enlightenment and clarification on the path to achieve a technical feasible product such as a clinical diagnostic assay.
IVD Product Development
IVD product development shares similar processes though varying technical details for different products. For example, clinical diagnostic assays can range from single marker assays to multiplex assays, from immunoassay to molecular diagnostics, from Point of Care Testing (POCT) devices to fully automated platforms, from Lineblot Immuno Assay (LIA), Enzyme-linked Immunosorbent Assay (ELISA), Enzyme-linked fluorescence assay (ELFA) to Chemiluminescent Immunoassay (CLIA), from Polymerase Chain Reaction (PCR)- to Next-Generation Sequencing (NGS)-based Molecular Diagnostics, from Clinical Trial Assay (CTA) to Companion Diagnostic Assay (CDx).
Helical Sphere will guide you throughout the product development process from concept to commercialization, including the technical aspect of product development under Design Control, and strengthen each weak link in your design and development process such as documentation under Design Control, risk analysis and management throughout the development phases, and product validation and quality control strategy.
Design Control
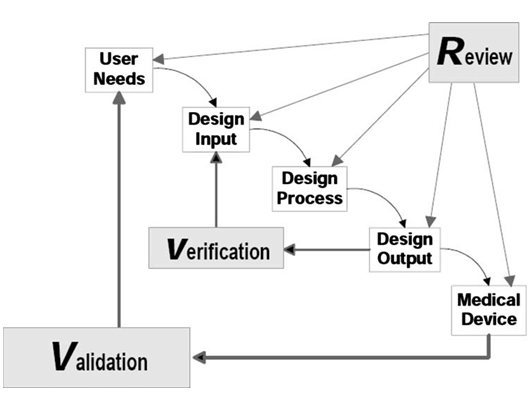
Beyond technology considerations, IVD product development is required by regulatory authorities to follow a certain rigorous process, namely Design Control. Each step, gateway activity, document generation, and file organization are to follow the defined process. However, there are very different approaches in working out the details, content of each document, and so on, and guidance from experts would shorten the time of trying to figuring out all by yourself, and make the process more effective and efficient that would save the organization much resource in getting a compliant design control process in place and well implemented.
Helical Sphere will help you in various ways depending on your current status and needs, from initiating a design control process, drafting design documents, reviewing design and documents, organizing and auditing design history file (DHF), and so on, to ensure a compliant design control process which is important for a successful regulatory submission and approval.

Raw Material Quality Control
Highly variable sources and natural characteristics of raw materials necessitate upstream material qualification and optimization as the most critical step to ensure high quality assay performance and lot-to-lot consistency, which is critical but many a time very difficult to achieve for IVD assays that utilize biological materials such as a mixture of proteins and other macromolecules.
Helical Sphere offers guidance on designing material qualification testing protocols, setting specifications, sourcing biological materials, investigating and optimizing raw materials for better and more consistent assay performance.
